How can we assist you?
Explore our network of country and industry based websites to access localized information, product offerings, and business services across our group.
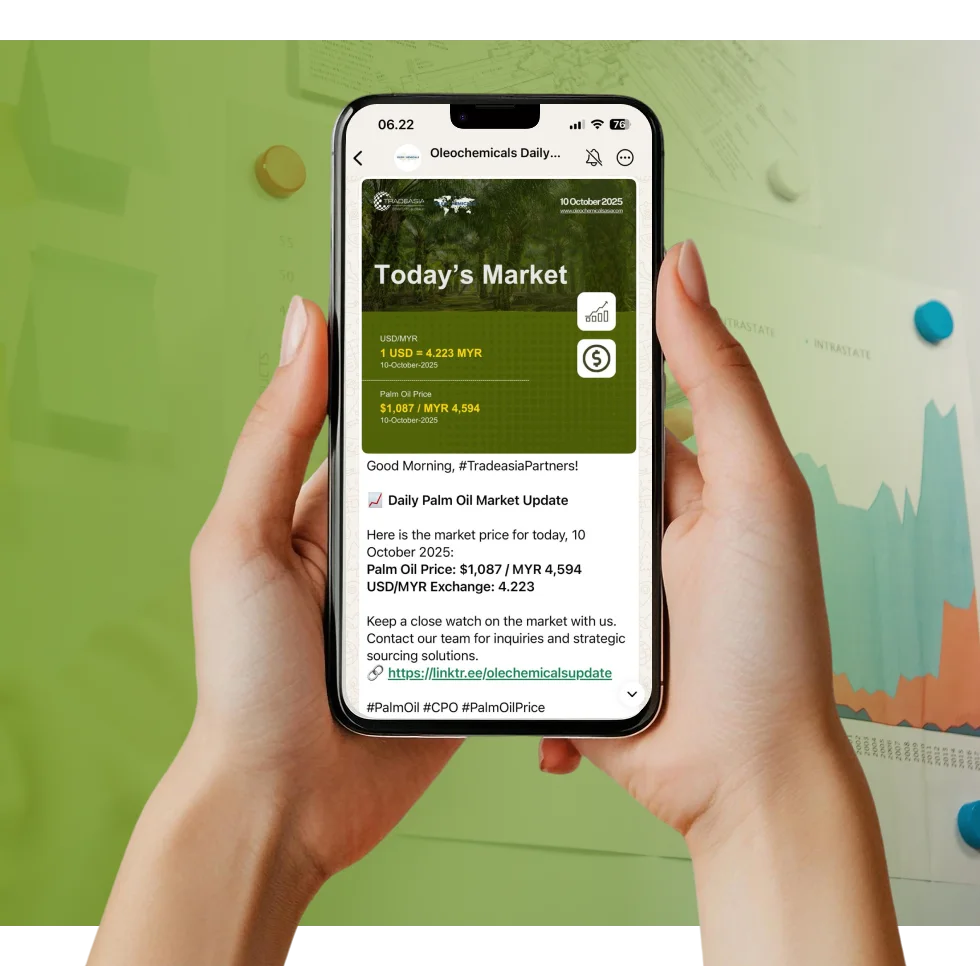
Access reliable chemical market information through our update channels.
Real-time Updates
Daily Updates
Log in to start sending quotation requests for any product.
Don't have an account? Sign Up Here
Log in now to access technical product documents from our product range.
Don't have an account? Sign Up Here
Home All Products Stearic Acid (C18 50% - 60%)
|
Origin |
: Indonesia |
|
IUPAC Name |
: Octadecanoic acid |
|
Cas Number |
: 57-11-4 |
|
HS Code |
: 3823.11.00 |
|
Formula |
: C18H36O2 |
|
Appearance Name |
: White Beads/Flakes |
|
Common Names |
: Octadecanoic acid |
|
Packaging |
: 25 kg Paper Bag - Loose Stuffed |
For more detailed information including pricing, customization, and shipping:
Brief Overview
Stearic acid, is present in high-fat sources from both plants and animals, and it belongs to the category of saturated fatty acids. Renowned for its positive health effects, it appears as a waxy white solid with a molar mass of 284.48 g/mol and a chemical formula of CH3(CH2)16CO2H. Naturally occurring in these fats are stearic, palmitic, and oleic acids, with the production of commercial stearic acid requiring nearly equal amounts of oleic and palmitic acids. Stearic acid is commonly found in nature either as an ester of fatty alcohol or as a mixed triglyceride with other long-chain acids. Notably, animal fats often contain a higher concentration of stearic acid compared to fats derived from plants.
Manufacturing Process
Fatty acids play a crucial role in stearic acid production, and the method depends on final product quality and raw material. Various protocols are used. Tallow and grease are common raw materials. The production involves two main steps:
a. Hydrolysis generates glycerin and fatty acids from raw ingredients (oil or fat), followed by the separation of the two products.
b. Separation includes the purification and separation of the fatty acid mixture in the second stage.
Paint Industry
Stearic acid stands as a highly effective wax modifier in the intricate craft of candle making. This non-toxic additive not only elevates the opacity and hardness of candles but also imparts a heightened whiteness, particularly accentuated in freestanding candles during warmer months. Beyond its aesthetic enhancements, stearic acid contributes to the structural integrity of candles, ensuring they maintain their shape. Moreover, it enhances the overall durability consistency and melting point, resulting in candles that are both resilient and long-lasting. The stability and exceptional shaping properties of stearic acid extend its utility to the creation of diverse and artistic craft products.
Detergent Industry
In the production of soap and cosmetics, including face wash, shampoo, beauty soaps, and shaving cream, stearic acid assumes a significant role. Its primary function involves serving as a thickening or hardening agent, aiding the soap in maintaining its structural integrity. Beyond its contribution to texture, stearic acid serves as a robust cleanser and functions as an emulsifying agent, facilitating the harmonious binding of oil and water.
Fragrance and Flavoring Industry
stearic acid is a commonly used as binder and flavoring component, strategically enhancing the flavor and texture of diverse food products. Its versatile applications encompass the creation of margarine, soft drinks, chewing gum, pastries, creamy spreads, and artificial sweeteners, among others. The deliberate inclusion of stearic acid in these formulations aims to elevate the sensory characteristics and visual allure of the products, contributing to a more delightful experience for consumers.
How can we assist you?
