Explore our network of country and industry based websites to access localized information, product offerings, and business services across our group.
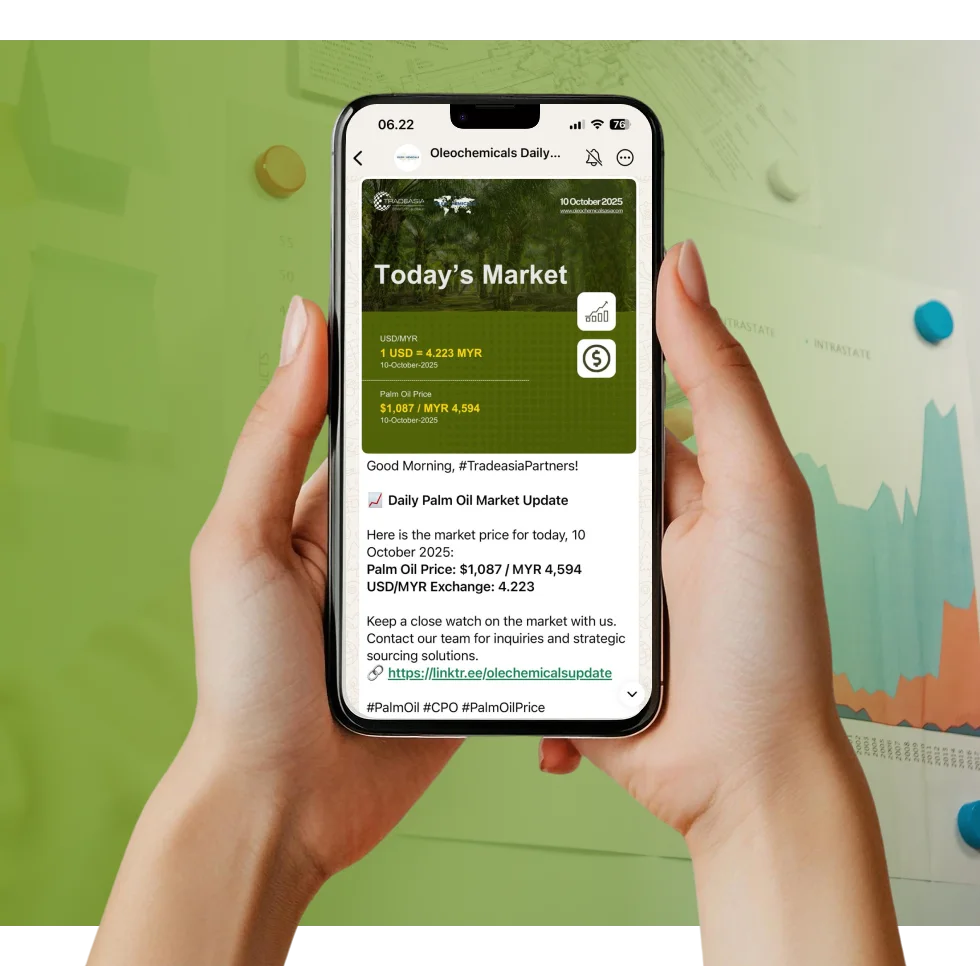
Access reliable chemical market information through our update channels.
Real-time Updates
Daily Updates
Log in to start sending quotation requests for any product.
Don't have an account? Sign Up Here
Log in now to access technical product documents from our product range.
Don't have an account? Sign Up Here
Home All Products Laundry Soap Noodles TOM 78% (45:45:10)
|
Origin |
: Indonesia |
|
IUPAC Name |
: N/A |
|
Cas Number |
: 143-07-7 |
|
HS Code |
: 3401.20.20 |
|
Formula |
: N/A |
|
Appearance Name |
: White Solid |
|
Common Names |
: Soap Chips |
|
Packaging |
: 25 kg paper bag |
For more detailed information including pricing, customization, and shipping:
Brief Overview
Soap noodles are produced by saponifying vegetable oils such as palm oil, coconut oil, olive oil, and/or animal fat (tallow) with sodium hydroxide. Considered essential components for soap manufacturing, these noodles are popular among both professional soap makers and hobbyists. Their versatility allows easy customization with pigments, fragrances, and various additives to create distinct soaps. The final soap product undergoes additional customization through molding, pressing, and stamping processes.
Manufacturing Process
Triglyceride molecules combine with sodium hydroxide in a process known as direct saponification, which is the most common method for making soap. Hydrolysis is the process by which fats and oils separate into their constituent fatty acids and glycerol. Next, sodium hydroxide is used to neutralize the fatty acids. The oil or fat is transesterified with methanol to yield methyl esters. After that, sodium hydroxide is used to saponify the resultant methyl ester, producing soap as well as methanol as a byproduct.
Detergent Industry
The process involves either placing the mixture in a worm screw or using rollers to create a thin sheet of soap. Subjected to high pressure, the liquid is stirred along the length of the screw and then extruded through a perforated endplate, resulting in multiple layers of soap. To form a single, continuous soap bar, the homogenized soap undergoes compression with a large worm screw extruder, commonly known as a plodder.
Various types of soap, including medicinal, high-lather, transparent, laundry, and toilet soap, can be crafted by utilizing different specifications of soap noodles.
