How can we assist you?
Explore our network of country and industry based websites to access localized information, product offerings, and business services across our group.
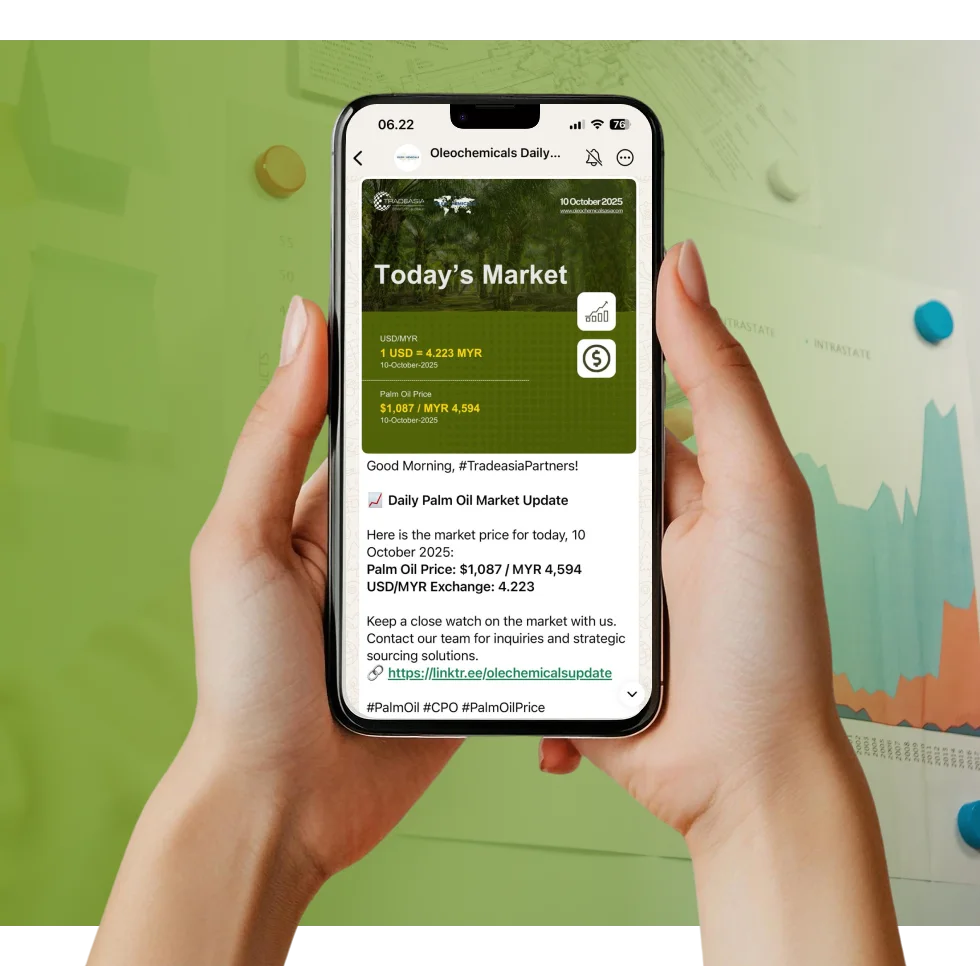
Access reliable chemical market information through our update channels.
Real-time Updates
Daily Updates
Log in to start sending quotation requests for any product.
Don't have an account? Sign Up Here
Log in now to access technical product documents from our product range.
Don't have an account? Sign Up Here
Home All Products Soap Noodles TFM 78% Min
|
Origin |
: Malaysia |
|
IUPAC Name |
: N/A |
|
Cas Number |
: 143-07-7 |
|
HS Code |
: 3401.20.20 |
|
Formula |
: N/A |
|
Appearance Name |
: White Solid |
|
Common Names |
: Soap Chips |
|
Packaging |
: 25 Kg Bag |
For more detailed information including pricing, customization, and shipping:
Brief Overview
Creating soap noodles involves the saponification process, employing sodium hydroxide to react with various vegetable oils such as palm, coconut, olive, and animal fats like tallow. Among the earliest soap varieties known, soap noodles are widely embraced by most soap manufacturers due to their efficiency in rapid soap production, especially when tweaking flavors, colors, and other ingredients. The final soap product can be customized further through additional techniques like pressing, stamping, and molding.
Manufacturing Process
The primary approach in soap manufacturing is direct saponification, where triglyceride molecules undergo a reaction with sodium hydroxide. Hydrolysis breaks down fats and oils into fatty acids and glycerol. Following this, sodium hydroxide neutralizes the fatty acids. The oil or fat undergoes trans-esterification with methanol, leading to the formation of methyl esters. These methyl esters are then saponified with sodium hydroxide to create soap, with methanol produced as a byproduct.
Detergent Industry
The mixture is put through rollers or a worm screw to produce a thin sheet of soap. High pressure is applied while the liquid is stirred throughout the screw's length, extruding numerous layers of soap through a perforated endplate. The homogenized soap is crushed with a huge worm screw extruder, often called a plodder, to produce several layers of soap.
To create many types of soap, such as laundry, toilet, medicinal, and high-lather soap, is facilitated by the specifications of various kinds of soap noodles.
How can we assist you?
