How can we assist you?
Explore our network of country and industry based websites to access localized information, product offerings, and business services across our group.
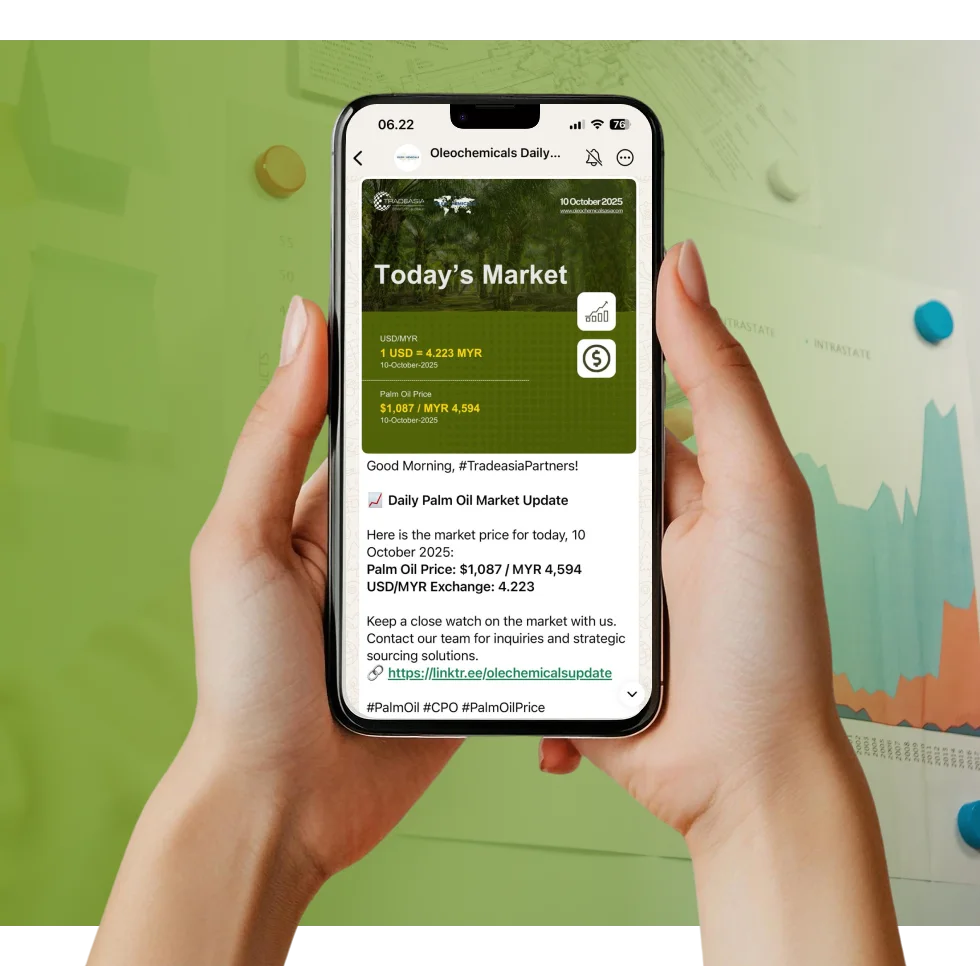
Access reliable chemical market information through our update channels.
Real-time Updates
Daily Updates
Log in to start sending quotation requests for any product.
Don't have an account? Sign Up Here
Log in now to access technical product documents from our product range.
Don't have an account? Sign Up Here
Home All Products Stearic Acid Triple Pressed (C18 38% - 45%)
|
Origin |
: Indonesia |
|
IUPAC Name |
: Octadecanoic acid |
|
Cas Number |
: 57-11-4 |
|
HS Code |
: 3823.11.00 |
|
Formula |
: C18H36O2 |
|
Appearance Name |
: White Beads/Flakes |
|
Common Names |
: Octadecanoic acid |
|
Packaging |
: 25 kg PP Bag |
For more detailed information including pricing, customization, and shipping:
Brief Overview
Stearic acid, frequently referred to as octadecanoic acid, is a popular and beneficial type of saturated fatty acid that may be found in both animal and plant-based fats. It is a waxy white solid with the chemical formula of CH3(CH2)16CO2H and molar mass of 284.48 g/mol. . In their natural form, oleic, palmitic, and stearic acids make up these fats; about equal amounts of oleic and palmitic acids are used to make a commercial stearic acid. Stearic acid is commonly found in mixed triglycerides with other long-chain acids or as an ester of fatty alcohol in nature. It's stated that animal fats often have higher stearic acid content than vegetarian fats.
Manufacturing Process
Fatty acids serve a purpose in the manufacture of stearic acid; the method used in this process is determined by the quality of the final product and the raw material used. Different protocols are used in accordance with these standards. The most widely used raw materials for the synthesis of stearic acid are tallow and grease. There are two main steps in the production process:
a. Hydrolysis : To produce glycerin and fatty acids, the raw ingredients (oil or fat) first go through hydrolysis. The next action is to separate the two final products.
b. Separation : The purification and separation of the fatty acid mixture are included in the second stage.
Paint Industry
In the practice of candlemaking, stearic acid turns out to be a useful wax enhancer. Beyond making candles harder and more opaque, this safe addition improves the brightness of freestanding candles and helps keep their shape, especially in warmer weather. Stearic acid additionally improves the melting point, consistency, and durability of candles in overall. It is a favored option for creating a wide range of artistic and creative products due to its solidity and shaping qualities.
Detergent Industry
The formulation of soaps and cosmetics, including face wash, shampoo, beauty soaps, and shaving cream, relies on stearic acid. The primary objective is to increase the soap's thickness or hardness, which maintains the soap's structural integrity. Stearic acid binds water and oil, keeping products smooth and creamy. It also works as an effective cleaner and emulsifier. This component is frequently added to shampoos, face cleansers, and shaving creams in order to make the most of its emulsifying and cleansing abilities.
Fragrance and Flavoring Industry
The food industry extensively employs stearic acid to augment the taste and consistency of various food products, utilizing it as both a binding agent and flavor enhancer. Its diverse applications encompass the creation of margarine, soft drinks, chewing gum, pastries, creamy spreads, and artificial sweeteners. The incorporation of stearic acid in these formulations is aimed at enhancing the sensory qualities of the products and enhancing their visual appeal to consumers.
How can we assist you?
