How can we assist you?
Explore our network of country and industry based websites to access localized information, product offerings, and business services across our group.
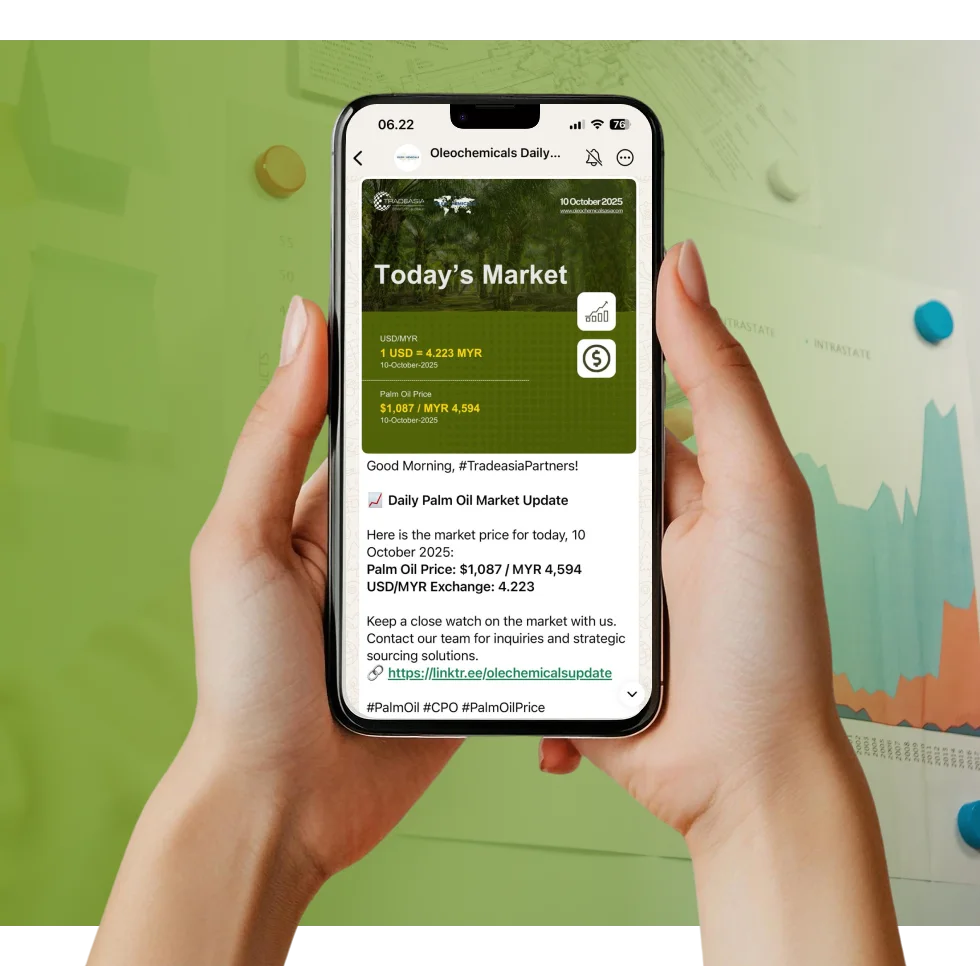
Access reliable chemical market information through our update channels.
Real-time Updates
Daily Updates
Log in to start sending quotation requests for any product.
Don't have an account? Sign Up Here
Log in now to access technical product documents from our product range.
Don't have an account? Sign Up Here
Home All Products Toilet Soap Noodles TFM 72% (90:10)
|
Origin |
: Indonesia |
|
IUPAC Name |
: N/A |
|
Cas Number |
: 143-07-7 |
|
HS Code |
: 3401.20.20 |
|
Formula |
: N/A |
|
Appearance Name |
: White Solid |
|
Common Names |
: Soap Chips |
|
Packaging |
: 25 Kg – Laminated Bags |
For more detailed information including pricing, customization, and shipping:
Brief Overview
To produce soap noodles, the saponification process is needed. It is usually done using sodium hydroxide to saponify vegetable oils, such as palm, coconut, olive, and/or animal fat (tallow). One of the earliest known varieties of soap are soap noodles. Most soap makers use soap noodles because they are simple to modify with flavors, colors, and additional ingredients to create soap. Additional procedures that can be applied to further modify the finished soap product include molding, pressing, and stamping.
Manufacturing Process
The prevalent technique for soap production is direct saponification, involving the reaction of triglyceride molecules with sodium hydroxide. Fats and oils undergo hydrolysis, breaking down into fatty acids and glycerol. Subsequently, the fatty acids are neutralized using sodium hydroxide. The oil or fat is trans-esterified with methanol to generate methyl esters, which are then saponified with sodium hydroxide, yielding soap with methanol as a by-product.
Detergent Industry
The mixture is treated either with rollers to make a thin sheet of soap or through a worm screw. Applying high pressure, the mixture is stirred along the screw and pushed through a perforated endplate, forming multiple soap layers. A big worm screw extruder, known as a plodder, compresses the mixed soap to produce a single long soap bar.
Different soap noodle specifications can be used to make various soap types, such as toilet soap, laundry soap, translucent soap, high-lather, medicated, and more.
How can we assist you?
